Cell Therapy Results Spur Pivotal Trial in Diffuse SCL Hand Function
Written by |
Fat-derived cells from adults with diffuse cutaneous scleroderma that are then injected under the skin of the hands led to clinically meaningful improvements in hand function, according to published data from the Phase 3 STAR I clinical trial.
The safety and efficacy of using these cells, called adipose-derived regenerative cells (ADRCs), will soon be evaluated in another Phase 3 trial, called STAR II (NCT05148598), by Paracrine, the therapy’s current developer. STAR II is expected to begin later this year and enroll up to 174 adults with diffuse cutaneous scleroderma.
“The consistency and durability of clinically meaningful improvement in patients with diffuse cutaneous scleroderma in this trial supports our hypothesis that a single treatment with ADRCs is safe and feasible, improves the ability of patients to perform daily activities, and improves their quality of life,” Dinesh Khanna, MD, at the University of Michigan, and co-principal investigator for both trials, said in a press release.
The STAR I trial findings were published in a study, “Adipose-Derived Regenerative Cell Transplantation in Systemic Sclerosis: Scleroderma Treatment with Celution Processed Adipose Derived Regenerative Cells- a RandomizedClinical Trial,” in the journal Arthritis & Rheumatology.
Hand problems are common among people with systemic scleroderma, also called systemic sclerosis (SSc), an autoimmune disease characterized by the buildup of scar tissue in the skin and internal organs.
Limited cutaneous scleroderma (lcSSc) is marked primarily by skin thickening, while those with diffuse cutaneous scleroderma (dcSSc) are more likely to have extensive skin scarring on the arms, legs, and body, and an increased risk of developing internal organ damage than those with limited scleroderma.
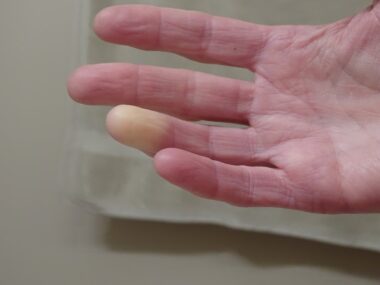
Hand impairment can significantly affect activities of daily living and quality of life in both types of conditions, which includes Raynaud’s phenomenon, indicated by reduced blood flow to the fingers.
“Despite the significance of hand dysfunction in patients with systemic scleroderma, there are few treatments with demonstrated effectiveness that specifically address this problem,” Khanna said.
Human adipose, or fat, tissue is a rich source of cells with the potential to treat SSc. When isolated from patients by liposuction using standardized clinical methods, these cells — a mixture of stem cells and other regenerative cells — are referred to as adipose-derived regenerative cells.
The approved Celution medical device isolates ADRCs from a patient and then injects the cells under the skin of the hands of the same patient, helping to reduce inflammation and promote healing.
Cytori Therapeutics (now Lorem Cytori), which first developed Celution, conducted the STAR I Phase 3 trial (NCT02396238) to evaluate the safety and efficacy of the therapy in 88 people, mostly women (85%), with SSc. Two injections of ADRCs were randomly assigned to 16 patients with lcSSc and 32 with dcSSc, while the remaining participants received a placebo.
The trial’s primary goal was a change in hand function at 24 and 48 weeks (about a year), assessed using a validated patient-reported outcome measure called the Cochin Hand Function Scale (CHFS). Additional outcomes included changes in the Health Assessment Questionnaire Disability Index (HAQ-DI) and the assessment of health-related quality of life (EQ-5D).
Although ADRC treatment was associated with improvements in CHFS that were numerically higher compared to placebo, the difference was not statistically significant at both time points across all participants and in each lcSSc and dcSSc subgroup.
The biggest difference was seen in those with dcSSc at 48 weeks, which showed a between-group improvement of 6.3 points. Among this group, 58% of those treated with ADRCs showed a minimal clinically important difference in hand function at 48 weeks compared to 26% in the placebo group.
HAQ-DI at 48 weeks was also numerically greater, but not statistically significant, for the ADRC-treated group overall, but again, mainly for those with dcSSc. Here, 63% of ADRC-treated dcSSc patients demonstrated a minimal clinically important difference in hand function at 48 weeks compared to 26% in the placebo group.
The researchers established a relationship between hand function and disability scores for those with dcSSc treated with ADRCs, whereby 52% of treated patients showed a minimal clinically important difference in both assessment tools compared to 16% with placebo. Assessment of quality of life was numerically superior at 48 weeks following ADRC treatment compared to placebo in all participants and in both subgroups.
Serious adverse events were reported in one individual in the ADRC group and five in the placebo group. The ADRC-treated patient was hospitalized due to respiratory complications deemed possibly related to either the liposuction or the injection procedure. The same patient experienced a second pneumonia eight months later.
Mild adverse events related to the liposuction procedure were reported in three ADRC-treated participants and three in the placebo group. Side effects potentially related to the injections were also mild and included injection site swelling and numbness.
“Based on these highly consistent and promising findings in patients with diffuse cutaneous scleroderma, we are planning to initiate the STAR II Trial by this summer to conclusively determine these benefits for this otherwise medically unaddressed condition,” Khanna said.
Early this year, the U.S. Food and Drug Administration granted an Investigational Device Exemption approval to Paracrine’s Celution medical device to conduct the STAR II trial, which has set a change in CHFS at 26 weeks (six months) as its primary goal.
“The initial STAR I trial, designed and conducted by our predecessor, was an important feasibility study that yielded valuable, actionable information on optimal patient selection and endpoints [goals],” said Alex Milstein, MD, chief clinical officer at Paracrine. “I would like to express our gratitude to the patients, investigators, and clinical trial staff who made this trial possible.”