Cytori Completes 48-Week Patient Follow-Up Visits in Scleroderma Trial
Cytori Therapeutics recently completed all 48-week follow-up visits in its STAR trial evaluating adipose-derived regenerative cells (ADRCs) for the treatment of patients with scleroderma-caused hand dysfunction. A total of 88 participants were enrolled, and the last patient’s 48-week visit was conducted earlier this week, the company said in a press release.
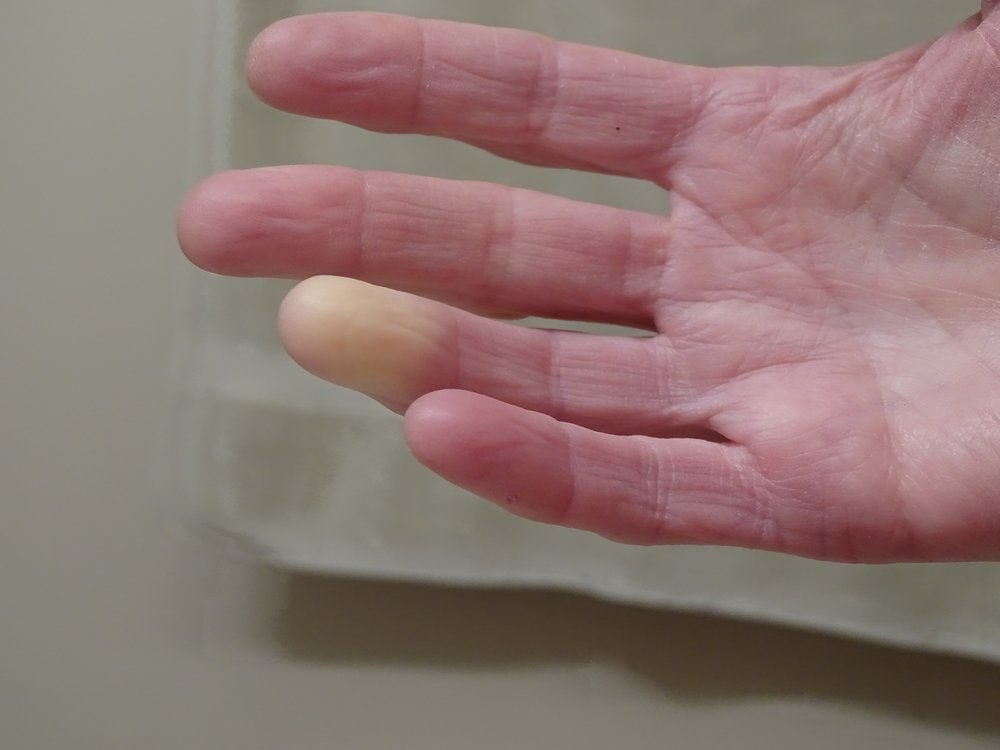
The ongoing STAR Phase 3 clinical trial (NCT02396238) is an FDA-approved placebo-controlled study evaluating the safety and effectiveness of Cytori’s Habeo Cell Therapy (ECCS-50) injected into the fingers of scleroderma patients who have experienced hand dysfunction due to the disease.
The treatment involves harvesting adipose (fat) tissue through small volume liposuction to isolate and concentrate ADRCs. Adipose tissue is known to be a unique reservoir of many types of cells that can contribute to the healing process.
In the trial, Habeo and the placebo were injected beneath the skin in the patients’ fingers. Final assessments were done after 48 weeks.
The study’s primary endpoint is the improvement in hand dysfunction up to 48 weeks after treatment as measured by the Cochin hand score compared to the placebo. Secondary endpoints include the study of Raynaud’s syndrome and health-related quality of life. Results from the study are expected in the coming months.
In the release, Cytori states that after investigators review the trial data, patients who were initially treated with the placebo might be offered the option to enroll in the crossover part of the trial. This means that if patients meet eligibility criteria and if they wish to do so, they can be treated with Habeo Cell Therapy.
Habeo Cell Therapy comprises a heterogeneous population of specialized cells, including stem, lymphatic, immune, and mesenchymal and endothelial progenitor cells, that can play a role in the healing process of patients with several conditions, including Raynaud’s phenomenon, a condition that affects the blood vessels primarily in the fingers and toes.
The STAR trial follows the SCLERADEC-I pilot trial (NCT01813279), which investigated the safety of subcutaneous injection of Habeo into the fingers of 12 patients with hand dysfunction associated with scleroderma. Results showed that patients treated with a single administration of Habeo Cell Therapy showed significant improvements in hand function and Raynaud’s phenomenon.
In December 2016, the company reported that this improvement was sustained at three years after the injection. Also, 83 percent of the patients had no symptoms of Raynaud’s in the weeks prior to each follow-up assessment, and maintained their improvement in hand strength and mobility scores since baseline.
In February 2017, the FDA’s Division of Industry and Consumer Education granted Cytori Therapeutics small business status for fiscal year 2017, meaning the company will pay reduced fees for regulatory filings that may arise from initiatives like the STAR trial.






