Improvement Patent Awarded to iBio for IPF And Other Fibrotic Disease Vaccines
New York based iBio, Inc. a biotech firm specializing in plant-based biotechnology for developing and manufacturing biological products and offering pharmaceutical product applications using its iBioLaunch platform, recently announced a new patent to aid in their development of a novel IPF therapy.
The iBioLaunch gene expression platform is a proprietary, transformative technology for the production of biologics including monoclonal antibodies, other therapeutic proteins and vaccines. It uses proprietary, transient gene expression in unmodified green plants instead of materials such as chicken eggs, bacteria, mammalian and insect cells, transgenic plants, and human blood plasma required by other systems, to produce important biologic pharmaceuticals.
iBioLaunch technology causes non-transgenic plants to rapidly produce high levels of target proteins. The plants are easily and reliably scaled-up in low cost, controlled growth facilities to produce large amounts of recombinant protein in the plant biomass. The technology is flexible, and has the capability to produce both biotherapeutics and vaccines.
The plant-based iBioLaunch platform is claimed to offer several significant advantages over other systems:
- Ability to synthesize complex proteins at which other systems have failed
- Simplicity, scalability and flexibility
- Reduced capital investment
- Lower operating costs
- Surge capacity
- Rapid production times gene sequence to factory-scale protein harvest
- Eliminates the need of bioreactors
- Low risk of contamination by animal pathogens
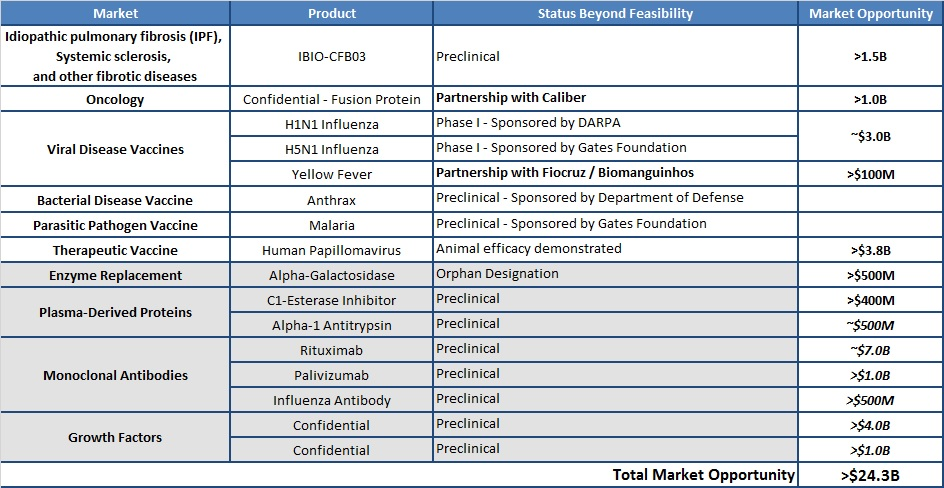
iBio has a strong IP/technology portfolio and has been well established by more than nine years of applications development and $100 million in funding. The company’s current pipeline includes:
- Monoclonal antibodies
- Interferons & cytokines
- Enzymes for replacement therapy
- Plasma-derived proteins
- Growth factors
- Protease inhibitors
- Clotting factors
- Vaccines
Advantages of the whole plant approach include lower operating and capital expenses compared to protein production using engineered microbial or animal cells in bioreactors. The iBioLaunch platform offers significant advantages over earlier plant-based methods that rely on transgenic plants or plant cell cultures. These include the speed at which different proteins can be expressed and produced at commercial scale, which presents a significant commercial advantage, as well as public safety benefits when vaccines for pandemic diseases are required. Other advantages include scalability, no risks of contamination by animal pathogens, and the ability to express complex proteins at which other systems have failed.
iBio’s technology has been validated in multiple vaccine and therapeutic product categories, and the company is developing a proprietary therapeutic product, IBIO-CFB03, for treating idiopathic pulmonary fibrosis (IPF), systemic sclerosis, and other fibrotic diseases in collaboration with Dr. Carol Feghali-Bostwick at the Medical University of South Carolina. IBIO-CFB03, produced using the iBioLaunch platform, is the first product candidate from this program designated for IND development.
IPF is a life-shortening lung disease with a rapidly progressing negative impact on quality of life leading to death within an average of three to five years after diagnosis. IPF has a worse survival rate than most cancers save for pancreatic cancer and certain lung cancers.
Systemic sclerosis is a disorder that affects connective tissue of skin and internal organs as well as blood vessel walls. Early diagnosis and individualized therapy can be helpful, but treatment of systemic sclerosis is limited to symptom management. No currently approved drug has been proven to arrest the underlying process or processes that drive progression of the disease.
Organ fibrosis is responsible for health care costs exceeding $10 billion per year, and the number of deaths due to fibrosis is estimated to be double those due to cancer. Organ fibrosis is responsible for nearly half of deaths in developed countries and results in significant physical, emotional, and financial burdens as well.
 Dr. Feghali-Bostwick leads a team of clinical and basic scientists whose research focus is on identification of novel targets for therapy and development of new anti-fibrotic strategies for scleroderma and other fibrosing conditions. Feghali-Bostwick and team are working on several aspects of scleroderma research: 1) identifying the mechanism used by novel factors to trigger fibrosis; 2) examining differences that may explain the occurrence of scleroderma in twins; and 3) conducting studies on a very promising drug which has already shown outstanding results in stopping and even reversing fibrosis in models of scleroderma.
Dr. Feghali-Bostwick leads a team of clinical and basic scientists whose research focus is on identification of novel targets for therapy and development of new anti-fibrotic strategies for scleroderma and other fibrosing conditions. Feghali-Bostwick and team are working on several aspects of scleroderma research: 1) identifying the mechanism used by novel factors to trigger fibrosis; 2) examining differences that may explain the occurrence of scleroderma in twins; and 3) conducting studies on a very promising drug which has already shown outstanding results in stopping and even reversing fibrosis in models of scleroderma.
Data published in 2012 by Dr. Feghali-Bostwick demonstrate the usefulness of specific endostatin-derived peptides for both inhibition and reversal of fibrosis in preclinical mouse models of fibrosis as well as in human skin. iBio has produced the active pharmaceutical ingredient using its iBioLaunch technology and has made the clinical development of this promising product a key priority.
Work in the Feghali-Bostwick laboratory has been supported by grants from the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases; SmartState SC Centers of Economic Excellence; and Biogen Idec, MA, Inc. Dr. Feghali-Bostwick also contributes to mentoring young physician and academic scientists.
iBio also offers proprietary products and product licenses to others, based on its iBioLaunch gene expression and iBioModulator thermostable immunomodulator protein platforms. iBio can provide collaborators with full support for turnkey implementation of its technology for protein therapeutics and vaccines. The iBioModulator platform is complementary to the iBioLaunch gene expression platform and is designed to significantly improve vaccine products with both higher potency and greater duration of effect.
iBio has formed a subsidiary company — iBio do Brasil Biofarmaceutical Ltda. — in Brazil, and has been collaborating with the Oswaldo Cruz Foundation (Fiocruz) since 2011 to develop a recombinant yellow fever vaccine based on iBio technology.
iBio this week announced that it has been issued a new U.S. patent expanding the company’s iBioModulator thermostable immunomodulator protein portfolio.
The new patent, serial number 9,012,199, entitled “Recombinant Carrier Molecule for Expression, Delivery, and Purification of Target Polypeptides,” grants iBio crucial exclusivity over new, modified carrier molecules derived from the company’s proprietary iBioModulator thermostable immunomodulator protein system and its use with one or more target proteins or antigens.
These proprietary carriers’ commercial significance is in their enabling the creation of high-performance vaccine candidates for difficult disease targets. The iBioModulator thermostable immunomodulator protein platform has been found to significantly improve the immune response to a vaccine in two important ways. It increases the intensity of the body’s initial immune response to a vaccine antigen (as measured by antibody titer), and it extends duration of the immune response.
Generating stronger immune response and prolonging immunity without booster inoculations adds significant value to a vaccine by reducing overall costs and logistical difficulties associated with its use. These capabilities may also enable governments or commercial producers to achieve and maintain protection of a larger proportion of a vaccine’s target demographic.
The iBioModulator thermostable immunomodulator protein platform, including the technology covered by this new patent, is applicable to biopharmaceutical products developed with iBio’s proprietary iBioLaunch plant-based gene expression system, and can also be used with other protein expression systems. iBio offers the benefits of its technology through licensing and also offers technology transfer arrangements for clients desiring fully autonomous manufacturing capability.
The new patent joins iBio’s other patents in this family in the US (8,173,408, 8,591,909), as well as patents in international markets including Australia, Canada, China, India, Germany, France, Ireland, Italy, Spain and United Kingdom.
Patents covering the iBioModulator thermostable immunomodulator protein platform are directly related to that part of iBio’s larger portfolio of intellectual property relevant to the development and manufacture of vaccines for a range of infectious diseases including plague, anthrax, human papilloma virus, and influenza. This immunomodulator portfolio includes vaccine product patents in the US (8,124,103, 8,277,816, 8,404,252, 8,945,580) and internationally.
Further information is available at:
https://www.ibioinc.com .
Sources:
iBio, Inc.
Medical University of South Carolina
Image Credits:
iBio, Inc.
Medical University of South Carolina